The COVID-19 pandemic has necessitated rapid, scalable and inexpensive diagnostic tests to identify symptomatic and asymptomatic cases. In edition two of QBRI Insights, the institute’s experts explained the rationale behind serology-based tests.
But how are these tests actually done? This week we talk about two popular methods, the lateral flow assay (LFA) and the enzyme-linked immunosorbent assay (ELISA), that have been authorized for emergency use by the U.S. Food and Drug Administration (FDA) for COVID-19. We also briefly discuss commonly observed neurological complications in COVID-19 cases.
Serological tests for COVID-19
Serology-based rapid tests detect antibodies in the patient’s blood, which show the response of the body’s immune system to a viral infection.
Lateral Flow Assays
The best-known application of a lateral flow assay is a pregnancy test, which detects a pregnancy-specific hormone. Similarly, lateral flow assays (LFAs) for COVID-19 rely on the detection of two antibody types generated by an infected individual against SARS-CoV-2, namely, immunoglobulin G (IgG) and M (IgM).
For COVID-19 diagnosis, the sample used is patient blood, which is applied at one end of the test device. The material within the device allows the blood to flow across and upon detecting antibodies against SARS-CoV-2, generates a colored line (for IgG or IgM or both). The control line indicates that the test has worked correctly.
Several studies have indicated reliable performance of LFA for COVID-19 diagnosis (1-7). A major advantage of LFA is its rapidity (results obtained in 10-15 minutes). In addition, it can be easily conducted by non-trained staff at the point-of-care and is simple to interpret with unambiguous colored bands.

Lateral Flow Assay for COVID-19
ELISA
As opposed to the lateral flow assay that provides a binary diagnosis (positive or negative), the enzyme-linked immunosorbent assay (ELISA) provides a quantitative result. ELISAs are commonly used tests in research laboratories to detect molecules of interest in a sample. On detection, a colorimetric or fluorescent reaction occurs and the intensity of the color/fluorescence indicates the quantity of the molecule present in the sample. In the case of COVID-19, the test molecules are antibodies generated against the SARS-CoV-2 in the patient blood. Accurately quantifying antibody concentrations using ELISA will help answer several research questions: how long do these antibodies last in an individual, and what is the threshold level of antibodies required to recover from a secondary SARS-CoV-2 infection?
While ELISAs are high-throughput and can be automated to test a large number of samples, a disadvantage is the requirement to be conducted in laboratories equipped with specialized instruments.
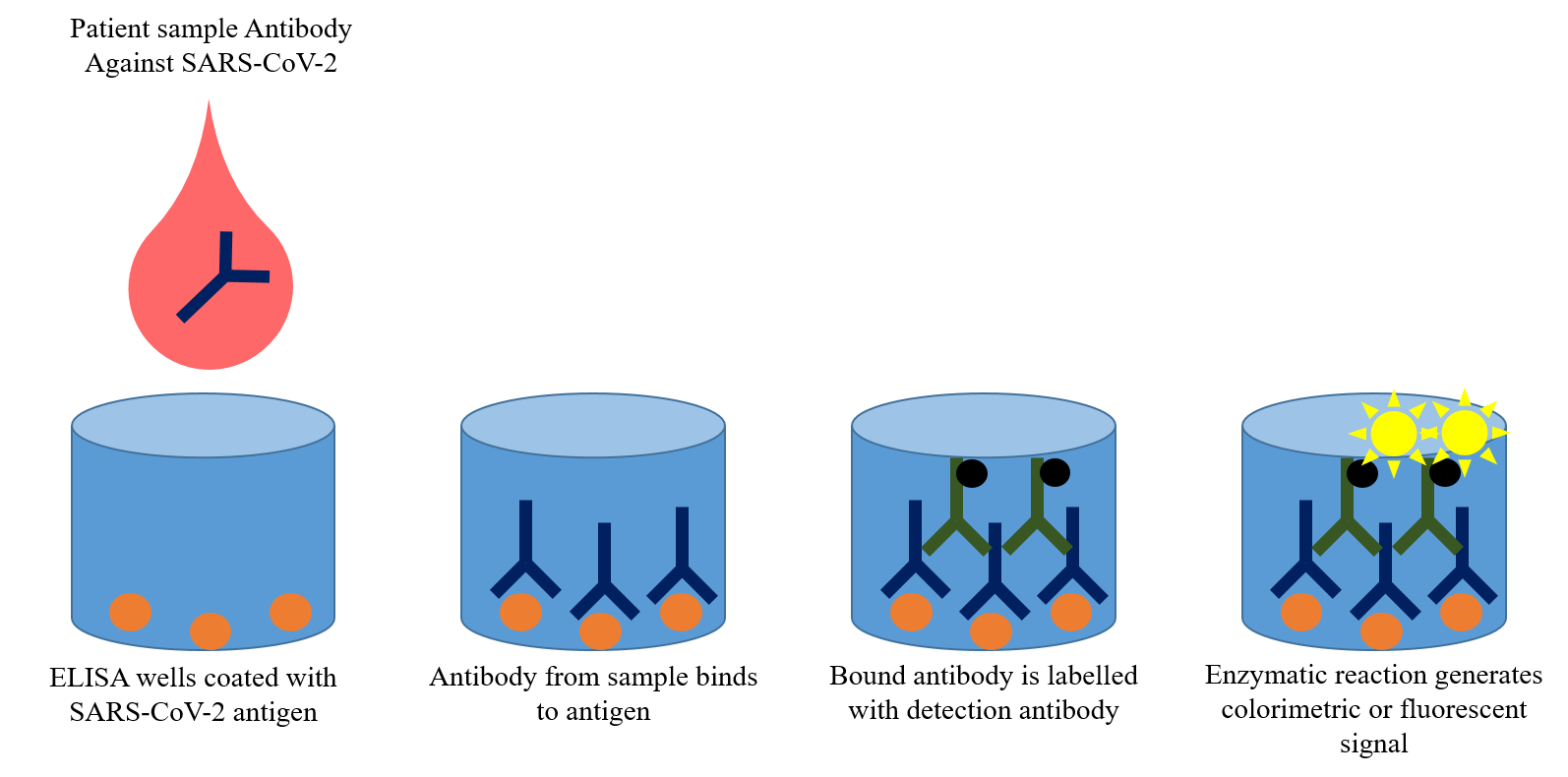
ELISA Test for COVID-19
To improve the diagnostic ability of serology-based tests, modified versions of the LFA and ELISA are being developed to also detect specific SARS-CoV-2 proteins instead of antibodies; however, these are still at an early developmental phase.
Due to their lower specificity and sensitivity compared to the reverse transcription polymerase chain reaction (RT-PCR), the gold standard diagnosis for COVID-19, serology-based tests can play a complementary role to test a wider population at a lower cost.
Apart from serology-based diagnosis of infected patients, the real advantage of LFA and ELISA tests lies in the identification of those who have recovered from an infection, i.e. convalescent individuals. These individuals can donate plasma, the fluid part of blood, for convalescent plasma therapy, an investigational treatment for severe cases of COVID-19.
These tests will also prove invaluable to determine the extent of herd immunity in the population in order to make critical decisions about relaxing social distancing and lockdown restrictions. Several countries are even considering an ‘immunity passport’ for convalescent individuals to be able to go back to work or travel.
Neurological complications of COVID-19
Although the main symptoms of COVID-19 are respiratory in nature with hepatic, intestinal and multi-organ failure, a large number of severe COVID-19 cases present with neurological complications (8, 9). The most common neurological manifestations are headache, dizziness, nausea, muscle pain and fatigue (8, 9). Loss of the ability to taste and smell have emerged as other common neurological features shown by patients (10). In addition, a reduced level of consciousness is observed in elderly and hypertensive COVID-19 patients (9).
Let’s try to understand what might be an explanation for these observations. The brain can be regarded as the control center of the body, rapidly transmitting instructions to peripheral organs via neuronal cells in the central and peripheral nervous system. Disruptions in the communication between the brain and vital organs can lead to organ failure and sensory disorders. Can SARS-CoV-2 infect brain cells and disrupt this communication?
Similar to the entry route of SARS-CoV-2 into human lung cells by binding to the ACE2 receptor – which was covered in edition three (https://www.hbku.edu.qa/en/news/qbri-insights-biology) present on the cell surface, ACE2 is also present on endothelial cells - the layer of cells forming a barrier between blood vessels and the tissues in our body.
This allows the virus to enter the blood circulation. Furthermore, the human brain and spinal cord are protected by special layers of cells, known as the Blood-Brain Barrier (BBB) or Blood-Cerebrospinal Fluid Barrier (BCF), which block the entry of foreign pathogens such as bacteria and viruses.
The exact cause of enhanced neurological disorders in COVID-19 patients is not clearly understood, although there is a strong likelihood that the virus has the ability to cross the BBB/BCF to reach the central nervous system (11).
Interestingly, brain cells also have ACE2 on their surface, although at lower levels compared to lung cells, thus providing entry to the SARS-CoV-2. Similar to findings with other types of coronaviruses, SARS-CoV-2-related effects on neurological functions may potentially be linked to acute respiratory failure or stroke observed in COVID-19 patients (12).
Several risk factors such as age, comorbidities and immune status govern the infection severity and, in turn, may possibly influence the neuropathology outcome of COVID-19 patients (13-15). Further research is required to shed light on the relationship between COVID-19 and neurological complications.
Section Contributors
Lateral Flow Assays: Dr. Afif Ben Mahmoud (Senior Research Associate, QBRI)
ELISA: Dr. Adviti Naik (Postdoctoral Researcher, QBRI)
Neurological complications of COVID-19: Dr. Vijay Gupta (Postdoctoral Researcher, QBRI)
Illustrations by: Dr. Afif Ben Mahmoud and Dr. Adviti Naik
Arabic text validation: Dr. Nour K. Majbour
Editors: Dr. Adviti Naik and Dr. Alexandra E. Butler (Principal Investigator, QBRI)
For references, please click here.
Related News