Breast cancer affects one in eight women, making it the most common cancer worldwide and in Qatar. It is also one of the leading causes of cancer-related death in women globally. The month of October is marked worldwide as Breast Cancer Awareness Month. In this edition, QBRI experts discuss the recent scientific advances in breast cancer diagnosis and treatment, in addition to highlighting the role of QBRI in supporting the fight against breast cancer through a comprehensive research strategy.
Biomarkers and Precision Medicine
Breast cancer is a heterogeneous disease - a trait that impacts the survival chances of the patient and their response to treatment. Only about 10 percent of breast cancer is hereditary or familial, whereby abnormal genetic risk factors are passed on from generation to generation within a family. In contrast, the majority of cases are sporadic in nature or with yet unknown genetic risk factors.
In light of this hurdle, the field of precision medicine is rapidly evolving and the diagnosis, treatment or prevention of breast cancer is complemented by taking into consideration specific individual gene alterations, the extent of gene alterations or mutation load, age, menopausal status and lifestyle. This approach could help doctors to personalize the treatment plan more accurately for each patient to favor a better disease outcome (Figure 1), in comparison to the one-size-fits-all approach.
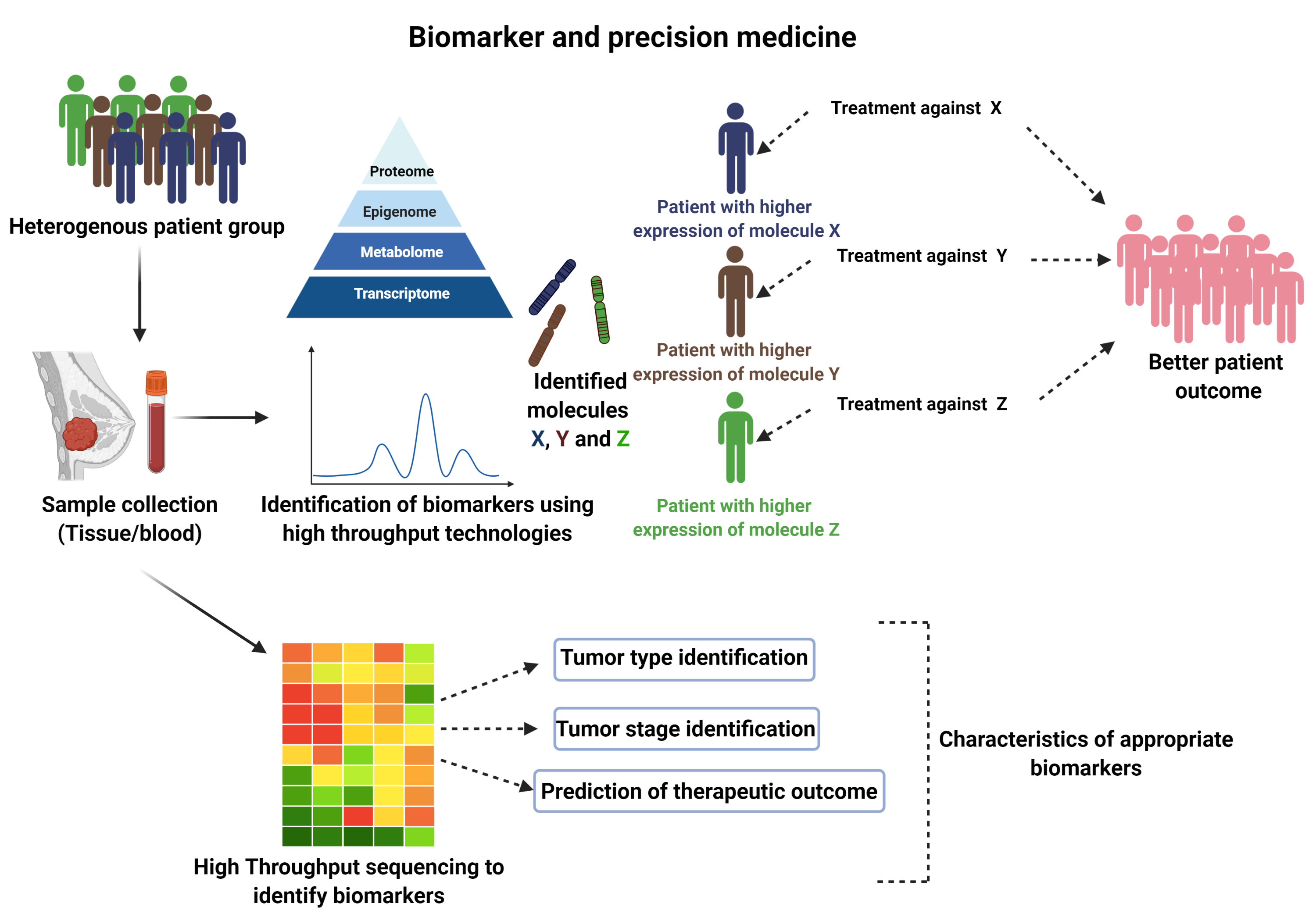
Figure 1. Schematic representation of the utilization of biomarker and personalized medicine in breast cancer therapy.
In cancer cells, various aberrant changes occur at the genetic, epigenetic, proteomic and metabolic levels that could disturb several ‘normal’ molecular or cellular processes regulating cell proliferation and survival, and cell motility, eventually leading to abnormal production of biological molecules.
Abnormal levels of these molecules could be used as biomarkers to accurately diagnose breast cancer stage and subtype, and as such could help to dictate the choice of treatment strategy (1-3). Biomarkers could include DNA, RNA, proteins, metabolites, and can be categorized as circulating tumor markers (CTMs) found in non-invasive biological samples such as blood or urine, or as tumor tissue markers (TTMs) (Figure 2).
Currently used diagnostic, prognostic and predictive tests are either based on the levels of different proteins in the breast tumor tissue, including the human epidermal growth factor receptor 2 (HER2) and estrogen (ER) and progesterone (PR) hormone receptors, or on mutation analysis (BRCA1/2 mutation) and multigene tests (oncotype DX, MammaPrint, ProSigna) that investigate the mutation/activity of a panel of genes to predict disease outcome and sensitivity or resistance to treatment. For example, alteration of HER2 expression is widely used to identify those patients who are most likely to benefit from treatment with trastuzumab, a specific inhibitor targeting HER2 (4). Various diagnostic, prognostic and predictive biomarkers utilized in breast cancer are depicted in Table 1.
Despite technological advances and our broadened understanding of the human genome, accurately detecting the molecular heterogeneity of breast cancer in the clinic remains challenging and expensive, requiring sophisticated molecular diagnostic laboratories.
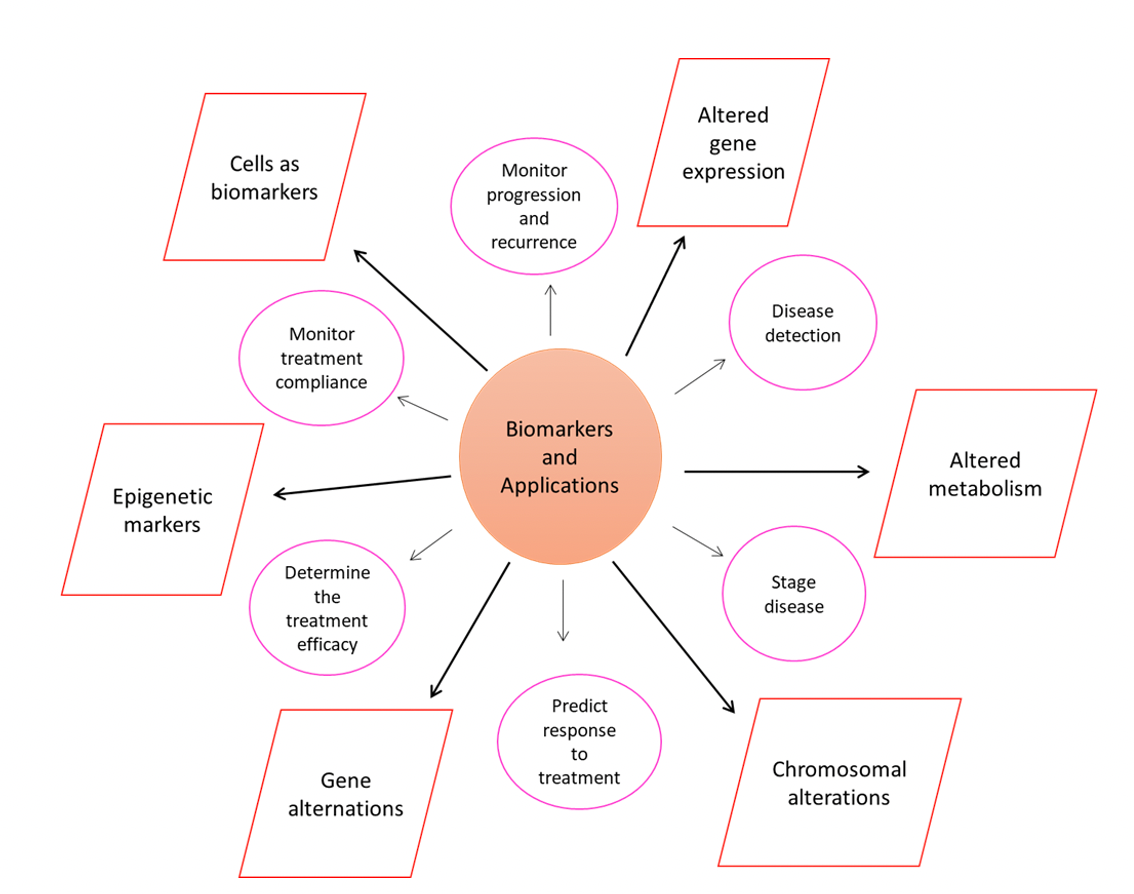
Figure 2. Schematic representation of commonly used biomarkers (boxes) and their applications (pink circles).
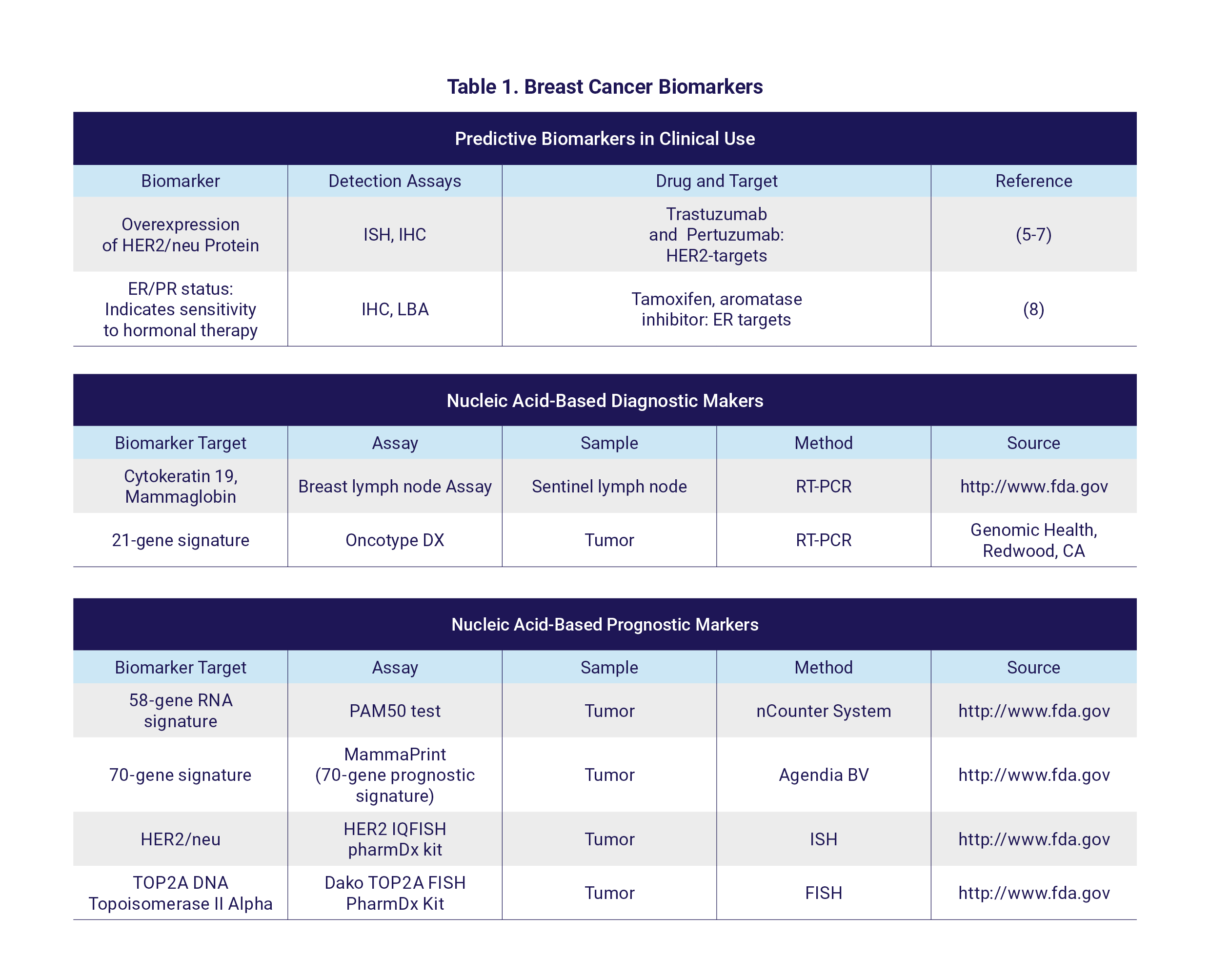
HER2, Epidermal growth factor receptor 2; (F)ISH, (Fluorescence) in situ hybridization; IHC, immunohistochemical staining; ER, estrogen receptor; PR, progesterone receptor; LBA, ligand binding assay; (RT-) PCR, (reverse transcription) polymerase chain reaction; FDA, Food and Drug Administration. PAM50 test: The Prosigna Breast Cancer Prognostic Gene Signature Assay.
Recent Breakthroughs in Treatment
Conventional breast cancer treatments to prevent the spread of tumor cells to other organ sites include surgery, radiation therapy, chemotherapy and hormonal therapies (Figure 3, conventional therapies). As breast cancer is a heterogeneous disease, its treatment is dependent on multiple factors, including cellular and molecular characteristics of the tumor, disease stage and history (9). Crucially, breast cancer patients have a greater than 90% survival rate when diagnosed and treated at an early disease stage.
Recent breast cancer therapies include drugs that specifically target molecules with important roles in cancer cell growth and survival, also known as targeted therapy (Figure 3, novel therapies). For example, alpelisib (PIQRAY, Novartis) specifically inhibits the phosphatidylinositol 3-kinase (PI3K) pathway that is involved in promoting tumor growth. Treatment with alpelisib prolongs progression-free survival in hormone receptor positive, HER2 negative, PI3KCA-mutant advanced breast cancer (10).
Another example is Talazoparib (TALZENNA, Pfizer) that specifically inhibits the integral DNA damage repair pathway (Poly-ADP-Ribose Polymerase inhibitor), thereby compromising the survival of cancer cells, and was approved in 2018 for treating locally advanced or metastatic HER2 negative breast cancer with BRCA1/2 mutations (11). Furthermore, the efficacy of histone deacetylase enzyme inhibitors (such as tucidinostat) that interfere with tumor-promoting signals and suppress tumor growth, is currently under investigation in clinical trials (12).
Recently, immunotherapy, a type of cancer treatment aimed at enhancing the immune response against cancer cells, has been recognized as a breakthrough for improving the survival of patients with certain cancer types. Several drugs have been developed to interfere with immune checkpoints, molecules serving as ‘off signals’ on immune cells to prevent immune cells reacting against normal cells. The use of such immune checkpoint inhibitors in cancer helps to unleash and activate the immune response against cancer cells.
One such drug is Atezolizumab (Tecentriq, Genentech), which specifically inhibits the immune checkpoint molecule programmed cell death-ligand 1 (PD-L1), and thereby promotes the recognition and attack of cancer cells by the immune system. It was approved by the FDA in 2019, in combination with chemotherapy for treating advanced breast tumors that do not express the hormone receptors or HER2, but have PD-L1 expression (13).
The biggest challenge in breast cancer treatment to date remains the targeted eradication of cancer cells with minimal non-specific adverse effects on healthy cells. Approaches such as adoptive T cell therapy, which involves the transfer of genetically engineered T lymphocytes that specifically recognize and kill tumor cells expressing a particular molecule and cancer vaccines, could have promising therapeutic benefits to eradicate tumor cells with high specificity and efficiency (14, 15). Lastly, tailoring targeted combination therapies based on predictive biomarkers and effective drug delivery through carrier systems, such as nanoparticles, have immense potential to improve breast cancer treatment (16).
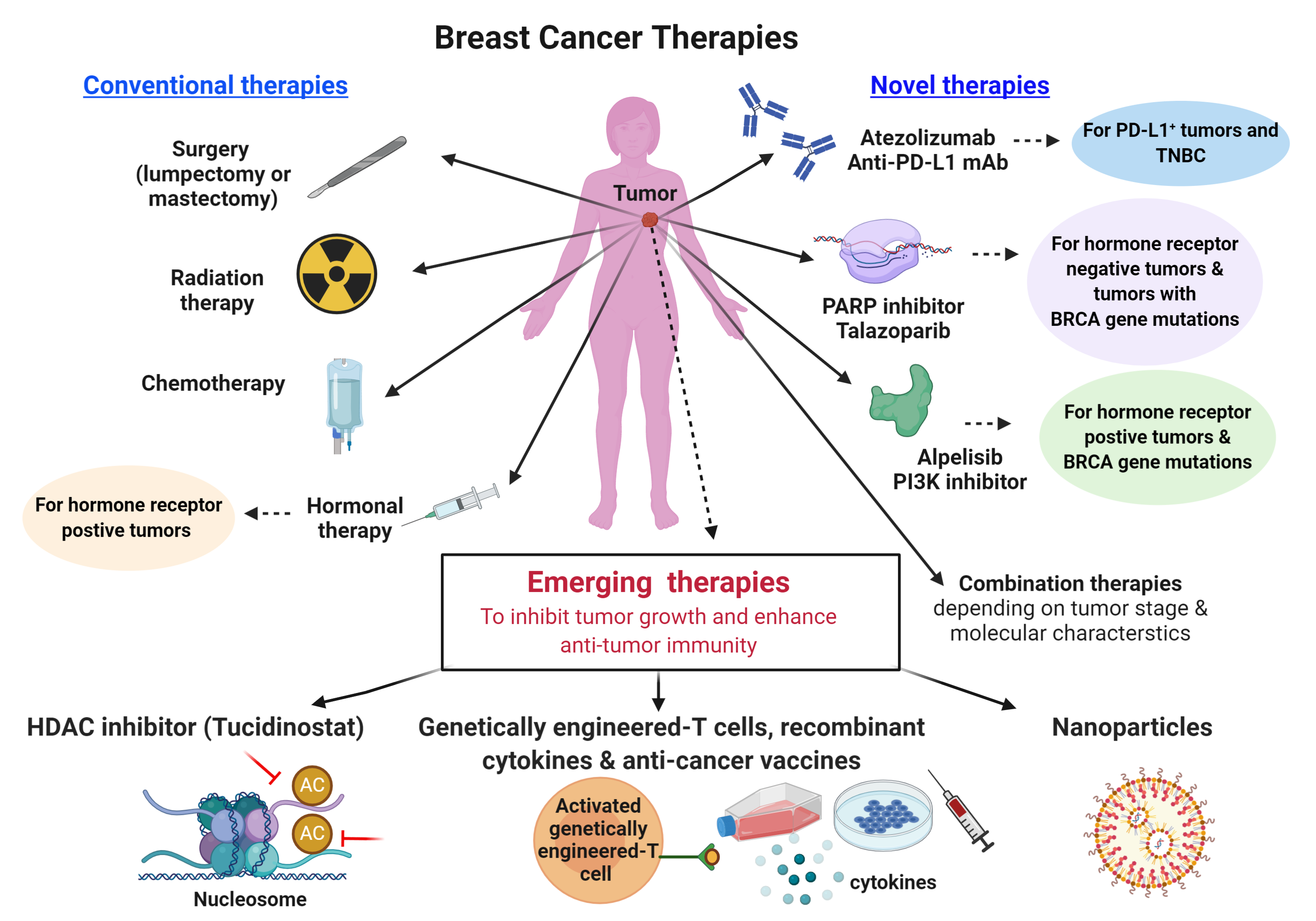
Figure 3. Currently available breast cancer therapies and proposed therapeutic strategies to improve clinical outcomes in patients who do not benefit from conventional therapies. TNBC, triple negative breast cancer; BRCA, BReast CAncer gene; PARP, poly (ADP-ribose) polymerase; PI3K, phosphatidylinositol 3-kinase; PD-L1, programmed cell death-ligand 1; HDAC, Histone deacetylase.
QBRI’s Breast Cancer Research Efforts
The cancer program at QBRI consists of several research teams with diverse expertise and research interests that can be put under two themes: biomarker discovery and mechanisms of resistance, and cancer immunology and immunotherapy.
The program employs state-of-the-art technologies and an integrative, multidisciplinary approach with potential implementation in personalized medicine with the ultimate aim of improving the survival of breast cancer patients.
Several research activities are focused on advancing the current knowledge of breast cancer risk factors, and to identify novel biomarkers for breast cancer in the Arab region. As such, gaining insight into the biology of breast cancer at the molecular and cellular levels can help to identify biomarkers for diagnosis, prognosis and prediction of treatment response.
For instance, the team recently performed comprehensive transcriptome analysis of breast tumors and identified a large number of protein-coding and non-coding RNAs that are differentially expressed in the context of breast cancer malignant transformation. Potential utilization of those genes as disease biomarkers and therapeutic targets is currently being investigated.
Another area of research in the cancer program pertains to the study of the interaction between cancer cells and our natural defense system - the immune system - and how cancer cells can escape recognition and killing by immune cells as well as how we can therapeutically intervene with this immune-escape. Our efforts in this area identified highly tumor-specific molecules that could be used to train immune cells to recognize and kill tumor cells that contain this molecule, or that negatively affect the activity of immune cells and therefore could be used as therapeutic targets to improve immune-mediated elimination of tumors.
To achieve its mission, the cancer program at QBRI has established strong collaborations with national and regional partners such as the Hamad Medical Corporation, Qatar and King Hussein Cancer Center, Jordan. In addition to its mandate to deliver excellence in research, the team leaders of the cancer program are also committed to capacity building in Qatar and are heavily involved in the teaching and research programs of the College of Health and Life Sciences at HBKU.
Section Contributors:
Biomarkers and precision medicine: Dr. Govinda Lenka (Postdoctoral Researcher, QBRI) and Dr. Varun Sasidharan Nair (Postdoctoral Researcher, QBRI)
Recent breakthroughs in treatment: Dr. Reem Saleh (Postdoctoral Researcher, QBRI) and Salman M. Toor (Research Associate, QBRI)
QBRI efforts towards breast cancer research: Dr. Julie Decock (Scientist, QBRI) and Dr. Nehad Alajez (Senior Scientist, QBRI)
Illustrations by: Dr. Govinda Lenka, Dr. Varun Sasidharan Nair, Dr. Reem Saleh, Salman M. Toor
Arabic text validation: Rowaida Z. Taha (Research Associate, QBRI)
Editors: Dr. Adviti Naik (Postdoctoral Researcher, QBRI), Dr. Alexandra E. Butler (Principal Investigator, QBRI), Dr. Julie Decock (Scientist, QBRI)
For references, please click here.
Related News