‘Normal life’ as we have always known it has collapsed worldwide due to COVID-19, which is caused by SARS-CoV-2. This week QBRI experts discuss why a vaccine is the only hope for resuming normality and highlight recent progress in multipronged approaches to SARS-CoV-2 vaccine development.
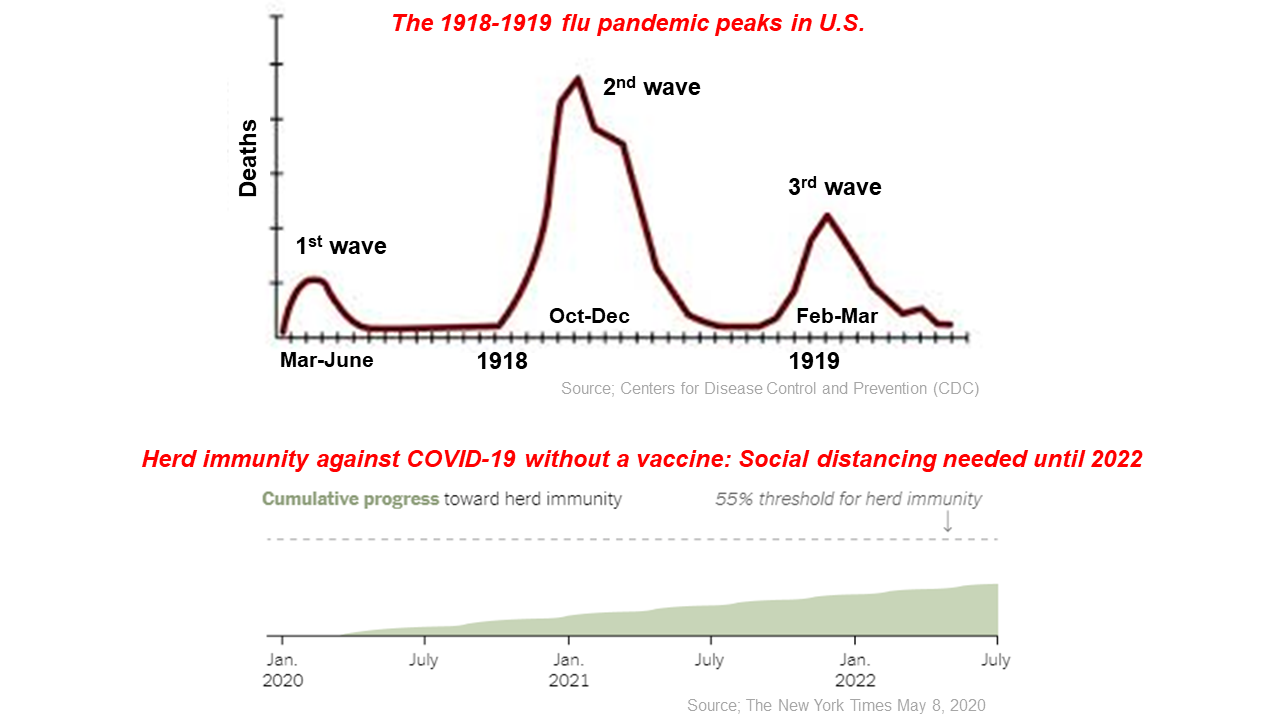
Pandemic patterns - A lesson from history
Mitigation measures such as social distancing, quarantining, and lockdown have been put in place in most countries, including Qatar, for the past two months. As some countries begin to reopen, the uncertainty still looms. SARS-CoV-2 is a previously unknown virus to us; however, we do have history to learn from.
The 1918-1919 H1N1 flu pandemic was the worst in recent history. About 500 million people, one-third of the world’s population at that time, were infected, leading to at least 50 million deaths worldwide (1). The pandemic was observed to have three outbreak waves. In the US, the first outbreak occurred in March-June 1918, the second in October-December of the same year, followed by the third in February-March 1919 (2).
In Spain, the same pandemic referred to as the ‘Spanish flu’ also manifested in three waves: May-July 1918, September-December 1918, and January-June 1919 (3). In both countries, the most severe outbreak in terms of mortality was during the second wave (3). Some reasons for the severity of the pandemic in 1918-1919 included World War I, with deployment of physicians to military service, limited medical care, and a lack of diagnostic tests (4, 5).
Since then, we have made significant advances in science, technology, and healthcare. Despite this, the Centers for Disease Control and Prevention (CDC) warned on April 21, 2020 that next winter a second wave of COVID-19 may occur with increased severity due to simultaneous epidemics caused by the seasonal flu virus and SARS-CoV-2 (6).
A recent mathematical modeling study published in the Lancet journal indicated that relaxing the strict social distancing and mobility restriction interventions would increase cumulative infected cases yet again because of incomplete herd immunity (7). It is speculated that the acquisition of herd immunity may be in 2021 or 2022 if there is no vaccine (8). In addition, mutations in the SARS-CoV-2 virus have serious potential implications for vaccine development. When restrictions start to ease, prevention measures need to be in place, including social distancing, behavioral changes such as handwashing and public awareness, as well as intensive testing and contact tracing. Until a vaccine arrives, we need to adapt to this ‘new normal’.
COVID-19 vaccine strategies
An effective vaccine against SARS-CoV-2 is our best hope of ending this pandemic. Vaccine development is an expensive and lengthy process, spanning 5-10 years of research, animal and human clinical trials, and analysis as per the traditional paradigm.
However, the current pandemic situation demands a more immediate response. Special paradigms are now being considered for COVID-19 to minimize the time spent on trials by parallel implementation of different phases, such as animal trials and phase I human trials.
As a result, some forecasts suggest that a vaccine for COVID-19 will be available early next year. The global landscape of COVID-19 vaccine developers includes the USA (46%), Asia and Australia (36%), and Europe (18%) (9). There are approximately 120 different SARS-CoV-2 vaccines currently being developed, including 75 now in use in clinical trials across the globe (https://clinicaltrials.gov/). At least six groups of scientists are testing different technologies; a few have started injecting formulations into volunteers while others are testing on animals (10).
Vaccines function by activating the immune system against either the whole virus or a particular fragment of the virus, but do not themselves cause disease. Seven teams are developing vaccines using the virus itself, in a weakened or inactivated form, which attempt to render the virus inactive by treatment with chemicals such as formalin.
Similar technology has been used in the past for the development of vaccines to protect against measles, polio and tuberculosis. Likewise, clinical trials in humans have started with attenuated versions of SARS-CoV-2 by Codagenix, USA. Additionally, 25 groups are working on viral vector-based vaccines, which are genetically modified viruses that produce coronavirus proteins inside the body and boost the immune response against SARS-CoV-2. This is a similar strategy to the development of vaccines to protect against Ebola, flu and measles.
Moreover, 20 teams are aiming to generate DNA or RNA-based vaccines using genetic instructions for the coronavirus spike protein, in order to stimulate an immune response. Although they take only a short time to produce, to date no DNA/RNA vaccines have been approved for humans. Spike protein-based vaccines have been developed in the past for other coronaviruses and tested in monkeys; however, no data is available from human trials.
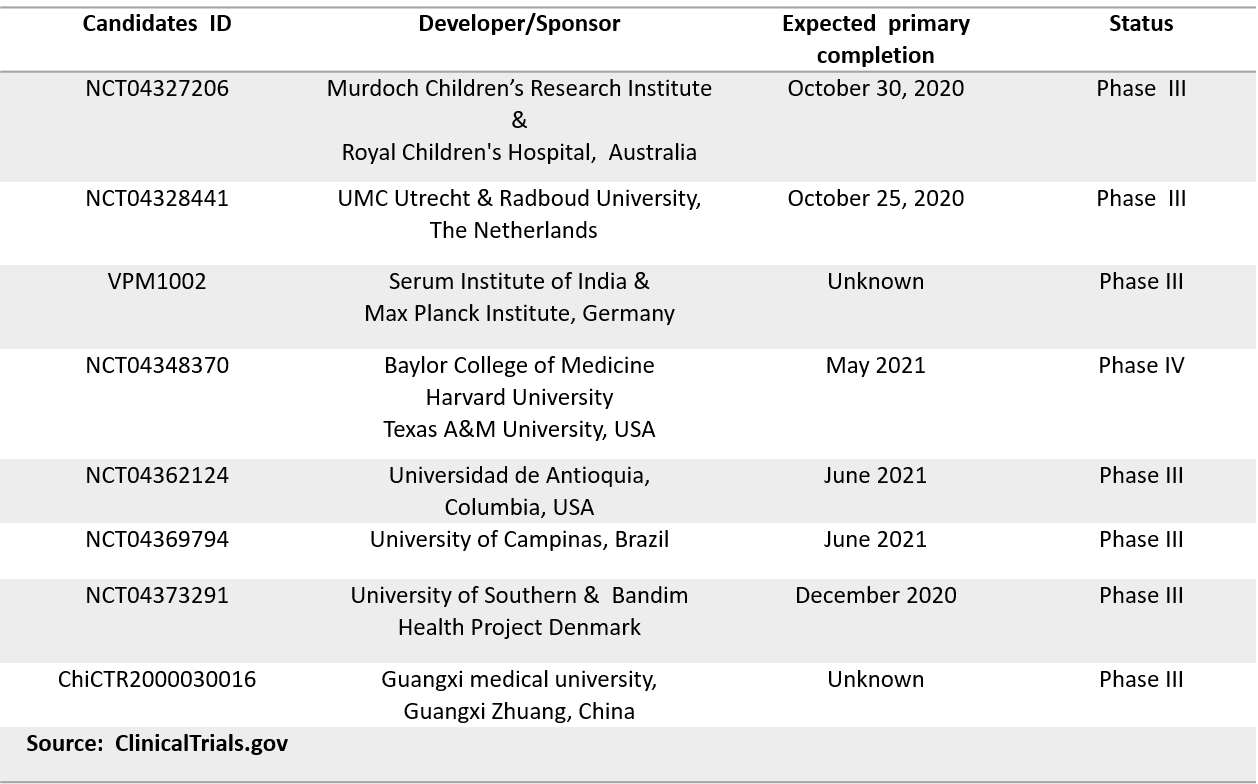
Several promising candidates have recently moved to early phase I development (Table 1). Moreover, the commonly used and well-known Bacillus Calmette–Guérin (BCG) vaccines against tuberculosis are being re-purposed for COVID-19 by eight research teams in more advanced phase III clinical trials (Table 2). According to recent reports, BCG may offer non-specific protection to healthcare workers against COVID‐19 (11).
When the optimal vaccine has been identified, the next challenge will be to produce enough of it to distribute globally. Thus, there is an urgent need for global collaboration to fight the COVID-19 pandemic.
Vaccine types against SARS-CoV-2
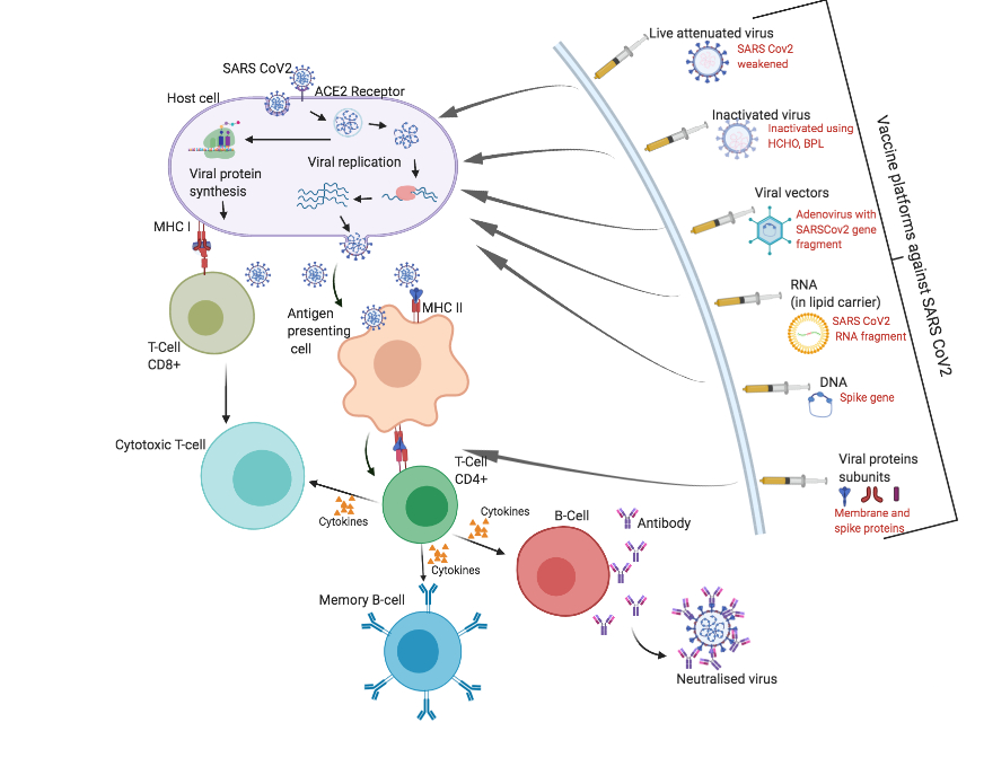
Adapted from Callaway, 2020. DOI: 10.1038/d41586-020-01221-y
Table 1. Clinical Trials for COVID-19 Vaccines
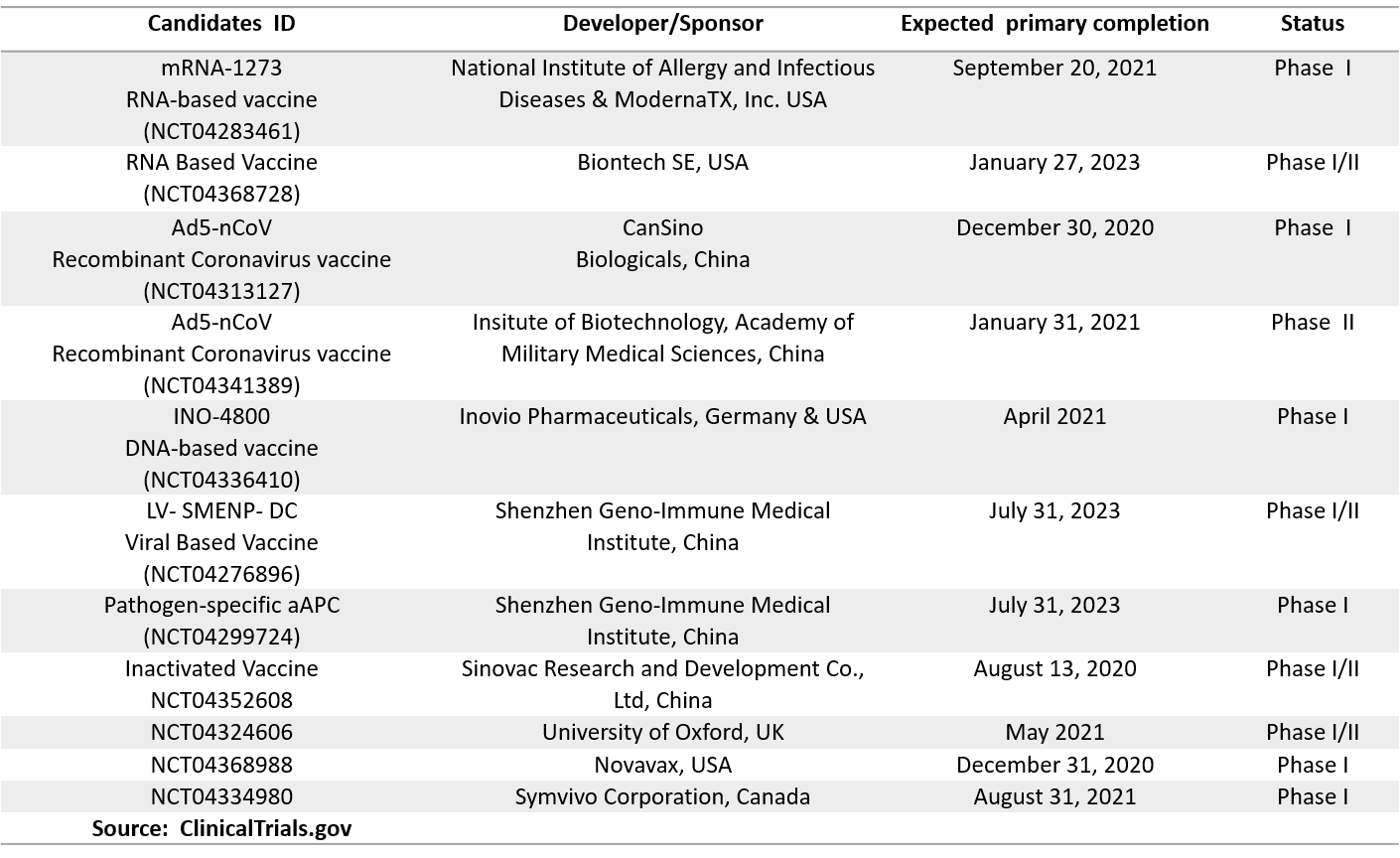
Table 2. Clinical Trials for Repurposing BCG Vaccines for COVID-19
Section Contributors
Pandemic patterns - A lesson from history: Dr. Yoshie Kobayashi (Postdoctoral Researcher)
COVID-19 vaccine strategies: Dr. Manjula Nandakumar (Senior Research Associate) and Dr. Ramesh Elango (Postdoctoral Researcher)
Illustrations by: Dr. Yoshie Kobayashi (Postdoctoral Researcher) and Dr. Manjula Nandakumar (Senior Research Associate)
Arabic text validation: Dr. Nour K. Majbour
Editors: Dr. Adviti Naik (Postdoctoral Researcher) and Dr. Alexandra E. Butler (Principal Investigator)
For references, please click here.
Related News