The global burden of neurological disorders is rapidly increasing, particularly due to the rise in the aging population worldwide. The Neurological Disorders Research Center (NDRC), one of the key research centers at Qatar Biomedical Research Institute (QBRI), housing research focused on neurological disorders of high prevalence in Qatar and the Pan-Arab region. The center’s work aims to understand the molecular mechanisms of diseases to facilitate diagnosis and therapeutic interventions for neurological disorders using innovative strategies. In this edition of the QBRI Insights newsletter, Dr. Yongsoo Park, scientist at the NDRC, discusses the potential application of certain biologically-derived nanoparticles, known as extracellular vesicles (EVs), in improving early diagnosis, intervention, and treatment of neurological diseases.
What are extracellular vesicles (EVs)?
EVs are lipid bilayer-enclosed nanoparticles that exist in all body fluids. EVs consist of exosomes and ectosomes, which are distinguished by several criteria including biogenesis, content, size, release pathways, and function (1) (Figure 1). Exosomes, ranging between 50 to 150 nm in diameter, originate from the endosomal pathway, a dynamic and interconnected system that allows for the trafficking and transfer of cargo between membrane-bound compartments, and are released during the membrane fusion of multivesicular bodies (MVBs) with the plasma membrane.
On the other hand, ectosomes, ranging from 50 nm to 1 μm in diameter, are directly secreted through the plasma membrane budding (1). EV structures were first described in 1946 as “pro-coagulant platelet-derived particles” (2) and Peter Wolf identified microvesicles as “platelets dust” in 1967 (3). More recent work, however, shows that EVs are secreted by different cell types and are involved in cell-cell communication through the horizontal transfer of biomolecules such as proteins and RNA (Figure 1).
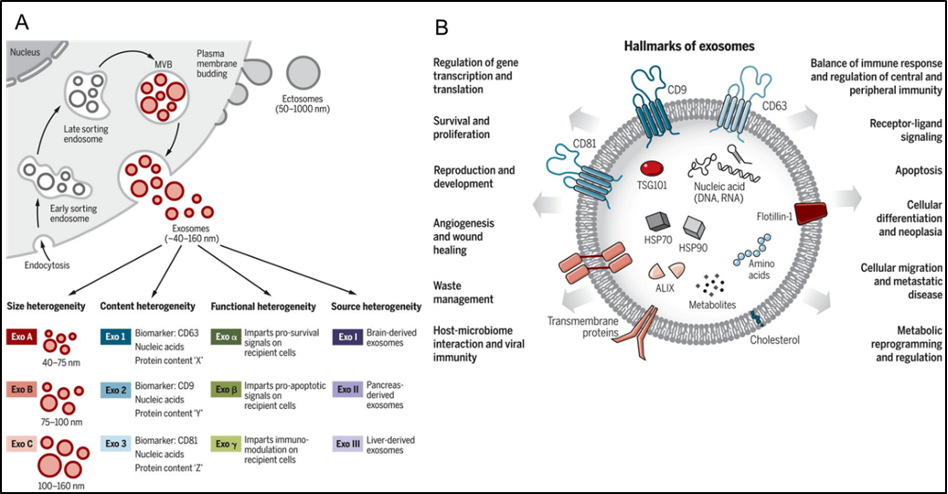
Figure 1. Extracellular vesicle (EV) in cell-cell communication. (A) Exosomes are heterogeneous in size, content, and biogenesis. (B) Exosomes and EVs are involved in cell-cell communication by stimulating receptor-mediated signaling and endocytosis into recipient cells. Adapted from (1).
EVs as biomarkers for neurological disorders
EVs play critical roles in health and disease and have a potential clinical utility as novel biomarkers to aid diagnosis as well as therapeutic targets for treatment (Figure 2). The contents of EVs can be non-invasive biomarkers for many different types of diseases. Because EVs can cross the blood-brain barrier (BBB), an additional semi-permeable boundary separating circulating blood and the brain, EV proteins and RNA are considered to be good biomarkers for neurodegenerative disease and neurological disorders, including Alzheimer’s disease and Parkinson's disease (4). Exosomes, isolated from the blood and cerebrospinal fluid (CSF) of Parkinson’s and Alzheimer’s patients, have been analyzed for differential expression of a class of small non-coding RNA molecules known as microRNAs (miRNA) (5). The changes in protein profiles of exosome in diseases also support the hypothesis that exosome RNA/proteins obtained from a liquid biopsy can be effective for the early detection of Alzheimer’s disease and Parkinson’s disease (6).
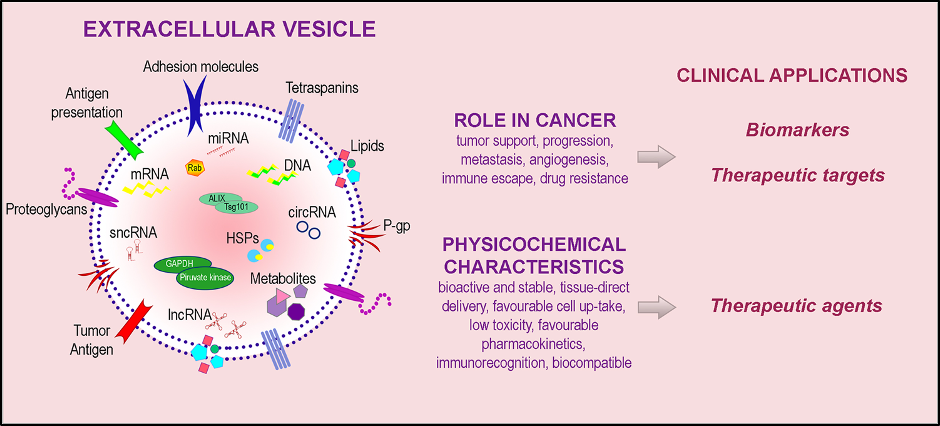
Figure 2. EV for clinical applications. EV cargo includes bioactive molecules on EV surface and molecular contents (DNA, mRNA, microRNA, long noncoding RNA, short noncoding RNA, circular RNA, metabolites, heat shock proteins, enzymes). EVs and their components play multiple roles in diseases and have unique physicochemical characteristics, thus holding a potential clinical utility as biomarkers as well as therapeutic targets and agents. Adapted from (7).
EVs and autism spectrum disorder
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental disorder characterized by persistent challenges in social interaction, speech and nonverbal communication, and restricted/repetitive behaviors. Due to the multifaceted nature of the disease, making a clinical diagnosis can be difficult. The specificity of EV biomarkers for ASD is currently unknown, but EVs may carry ASD-specific RNA/proteins that reflect the severity of ASD.
For the last 20 years, Dr. Park has been using advanced interdisciplinary techniques including electrophysiology, cell biology, imaging, biochemistry, and biophysics in molecular neuroscience to translate basic research into diagnostic and therapeutic strategies for neuropsychiatric disorders. The research goals of Dr. Park’s group at QBRI involve the identification and validation of ASD-specific biomarkers that originate from EVs in blood samples of ASD patients by applying a combined genomics and proteomics targeted approach for early diagnosis of ASD (Figure 3). Dr. Park’s research team is also conducting electrophysiological studies for a functional assay to validate patient-specific human induced pluripotent stem cells (hiPSC)-derived neurons, using calcium imaging, whole-cell patch clamping technique and micro-electrode array (MEA), in order to model neurodevelopmental and neurodegenerative diseases (8). Moreover, they are interested in dissecting the molecular mechanisms of vesicle fusion involved in neurodegeneration and neurodevelopmental disorders (9, 10).
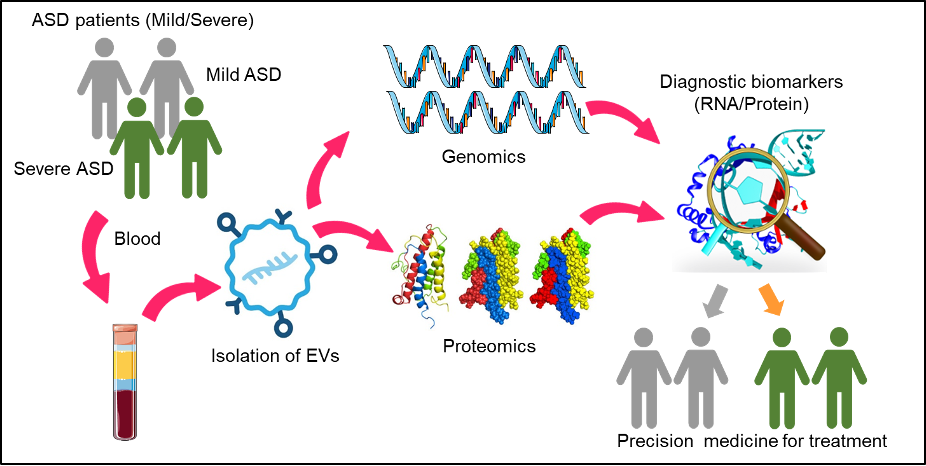
Figure 3. Schematic representation of the research goals of Dr. Park’s group. EV isolation from plasma of ASD patients using size exclusion chromatography for high purity. Sorting neuronal EVs and exosome from plasma using flow cytometry. Genomics and proteomics approaches including liquid chromatography with tandem mass spectrometry to identify specific and novel protein and mRNA biomarkers for ASD as part of a precision medicine application to predict the severity of ASD.
Contribution and illustration by: Dr. Yongsoo Park (Scientist, QBRI)
Editors: Dr. Adviti Naik (Postdoctoral Researcher, QBRI), Dr. Prasanna Kolatkar (Senior Scientist, QBRI)
For references, please click here.
Related News