News
QBRI Insights: Our Natural Defense Against COVID-19
20 Apr 2020Social distancing and quarantine are causing many of us to feel anxious and wonder why such tough measures have been put in place. Naturally, the question everyone is asking is: when will this end?
Humans possess a complex natural defense army, the immune system, which responds to viral infections. In most survivors, the immune system wins the fight, clears the infection, and the recovered patient is now immune to future infections.
However, the scenario is not as straightforward when a virus previously unknown to humanity, such as SARS-CoV-2, the virus strain that causes COVID-19, strikes. This week, QBRI experts reiterate the necessity for the current public health measures, discuss methods to detect the immune response for COVID-19 diagnosis, and assess the possibility of leveraging the successful immune response in recovered cases as a means of treatment.
Epidemiology: Mitigation vs Herd Immunity?
The main known mode of transmission of COVID-19 is by droplets generated when an infected person coughs, sneezes, or speaks. When these infectious droplets are internalized by an unaffected individual through the mouth, nose or eyes, their risk of infection increases.
In order to develop strategies against a previously unknown infectious disease, it is important to understand the extent of human-human transmission of the pathogen at an early stage.
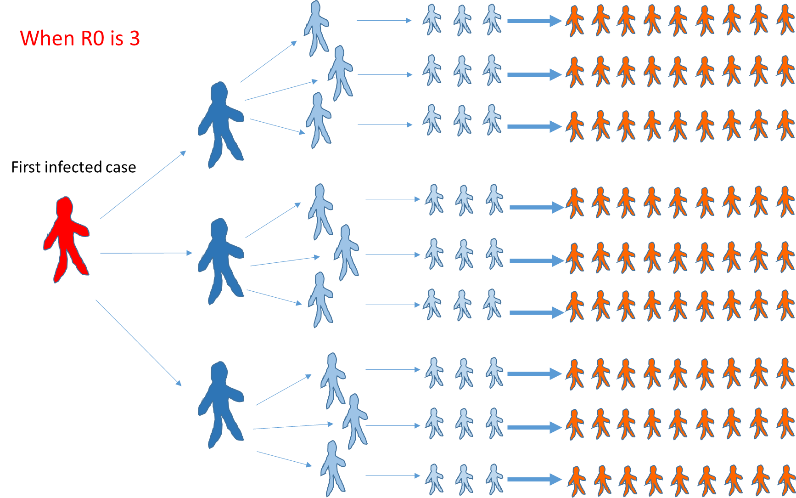
The basic reproduction number R0 (R nought) represents the infectious capacity of the virus, which is the average number of secondary cases generated by one infected individual in a fully susceptible population (1,2). When R0 is higher than 1.0, long-lasting person-person transmission will continue. In several reports, R0 of COVID-19 ranged from 2 to 5 (1,3-5) while the World Health Organization (WHO) reported R0 of 1.4 to 2.5 (6).
To explain this simply, how many people would hypothetically be infected in five days from one infected case when the R0 is 3? The answer is 81 cases! This one case, especially if asymptomatic (showing no symptoms), is termed a ‘super-spreader’.
In South Korea, a majority of the country’s positive cases were linked to one super-spreader. This phenomenon has resulted in the exponential increase in COVID-19 cases globally in a short time span. For comparison, the influenza virus has an R0 of 1.3.
If we let the coronavirus run its course naturally without tough public health mitigation measures, we predict that most survivors will build their natural defense and be immune to future infections, with an estimated mortality of 1-3%.
This leads to the interesting concept of “herd immunity” that occurs when a large proportion of the population (generally greater than 50%) acquires immunity against the disease. It means that the “herd” is immune; however, not all the individuals within it are.
A 1% fatality rate seems low but a global population of close to 8 billion equates to 80 million deaths worldwide. Additionally, about 10% of COVID-19 cases need hospitalization with about 5% requiring ventilation and, at present, no medical system can handle such emergency pressure.
Until we have an effective vaccine or treatment, the option of herd immunity is unthinkable (7). However, considering the economic and social consequences of strict mitigation, slowing down the spread of the virus while at the same time developing group immunity in a controlled manner is a potential strategy.
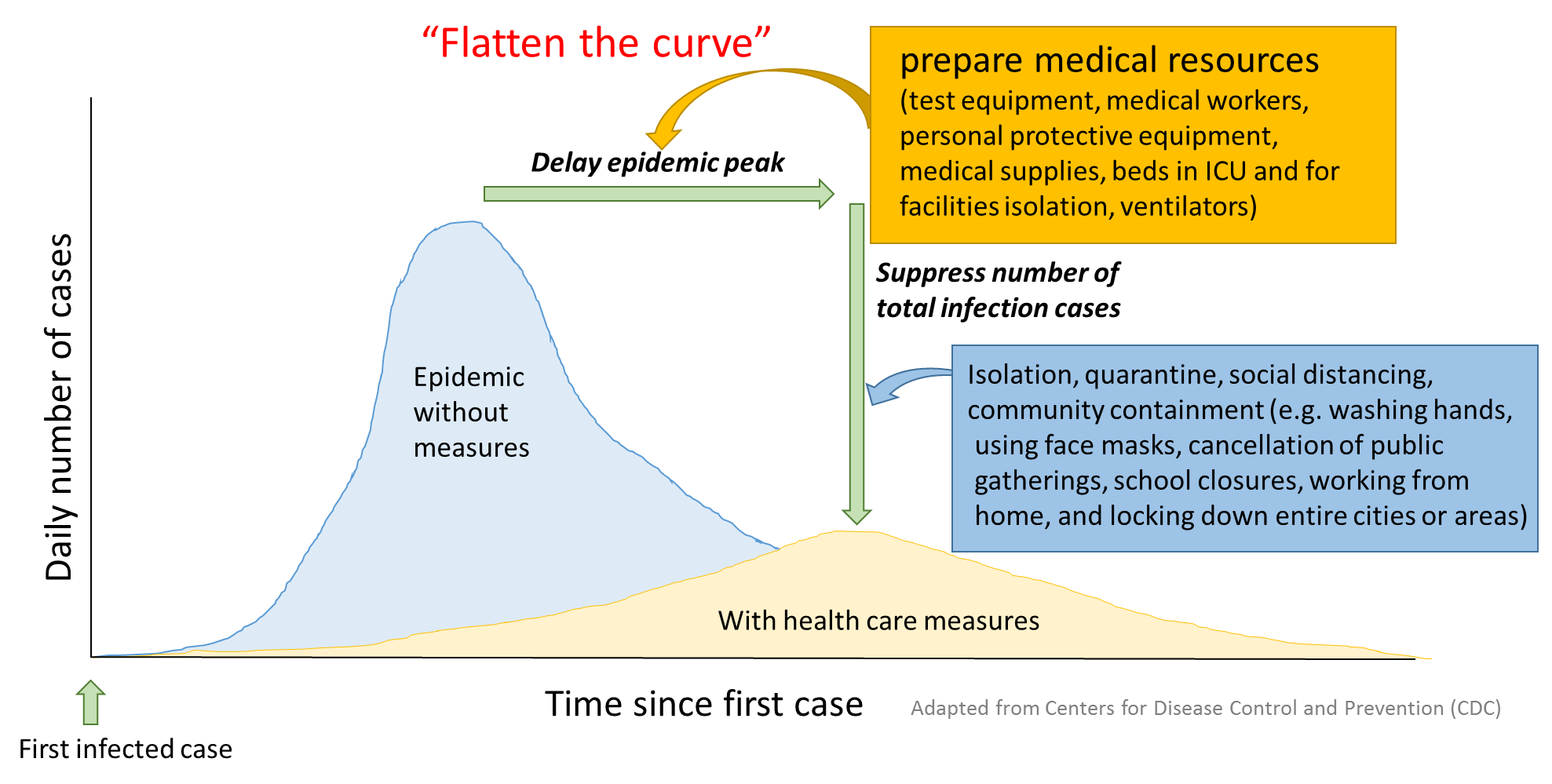
The current strategy in most countries is to “flatten the curve”, which means spreading the rate of infection over a longer period of time through quarantine, social distancing, remote working and education, and limiting the size of gatherings. These measures proved effective during the SARS epidemic in 2003 (8).
This can buy time for national public health authorities to bolster their medical facilities and increase testing capacity and for researchers to develop vaccines and treatments so that when the second wave of infections strikes after easing the restrictions, the healthcare response can be rapid and robust. This is why, for now, everyone needs to stay safe at home.
Diagnostics: Serology-based tests for COVID-19
Of the many methods proposed to diagnose COVID-19, serological tests are among the most prominent.
Serological tests measure the levels of antibodies in the blood generated by our immune system in the fight against an infection (9). In the case of COVID-19, those antibodies are specifically generated by our innate immunity against SARS-CoV-2 to neutralize the viral infectivity.
In contrast to RT-PCR (polymerase chain reaction with real-time reverse transcription), the gold standard test for COVID-19 diagnosis, serology tests measure the immune response against the virus in the blood rather than the virus genetic material in respiratory secretions. Although not yet recommended by the WHO as diagnostic tests for COVID-19, serology tests can still play a critical role in our fight against the pandemic, including containing the spread of the virus (10).
Serology testing can identify convalescent individuals - those who have already developed an immunity against the virus and therefore may no longer be susceptible to infection (11). These individuals may be able to return to work to support their country’s economy.
Unlike the RT-PCR test, serological testing doesn’t require highly skilled personnel, sample collection is more convenient, and antibodies are more stable during collection, transport, storage and testing. When dealing with a pandemic with potentially millions of people at risk of infection, serological testing is the preferred approach (12).
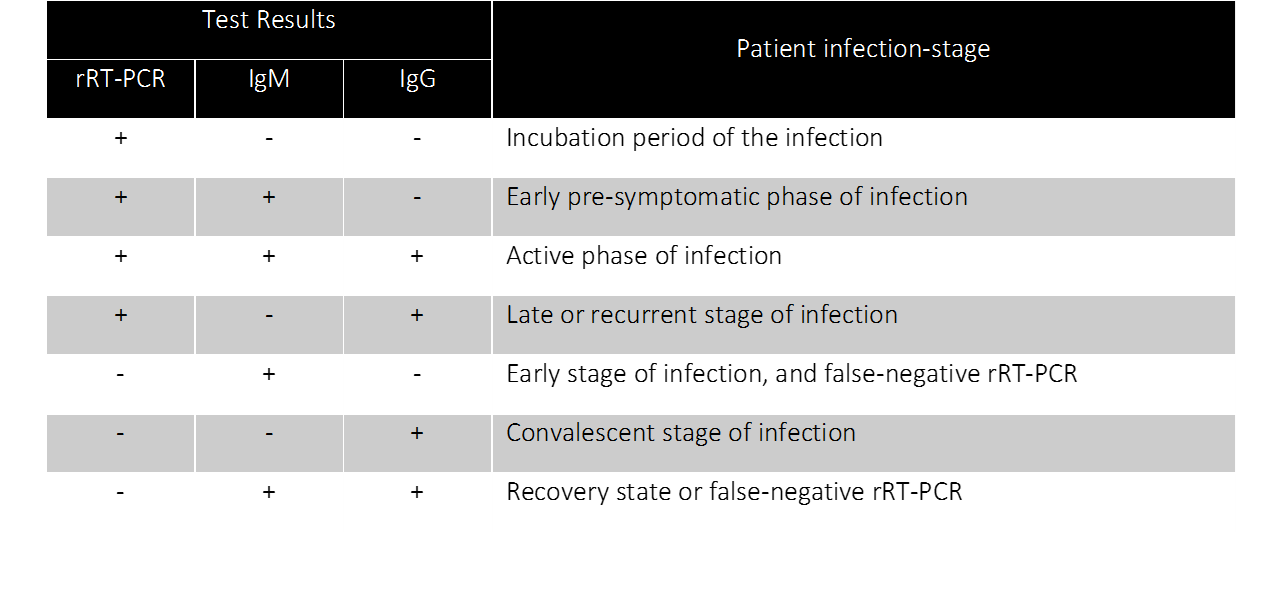
To be able to properly implement serological testing in the fight against COVID-19, we need to understand how to interpret the antibody levels in the blood. The relevant antibodies are of two types: IgM antibodies, the first to be generated in an immune response and whose levels quickly decline, and IgG antibodies, whose levels begin to rise a few days later and last longer in the blood (13-15).
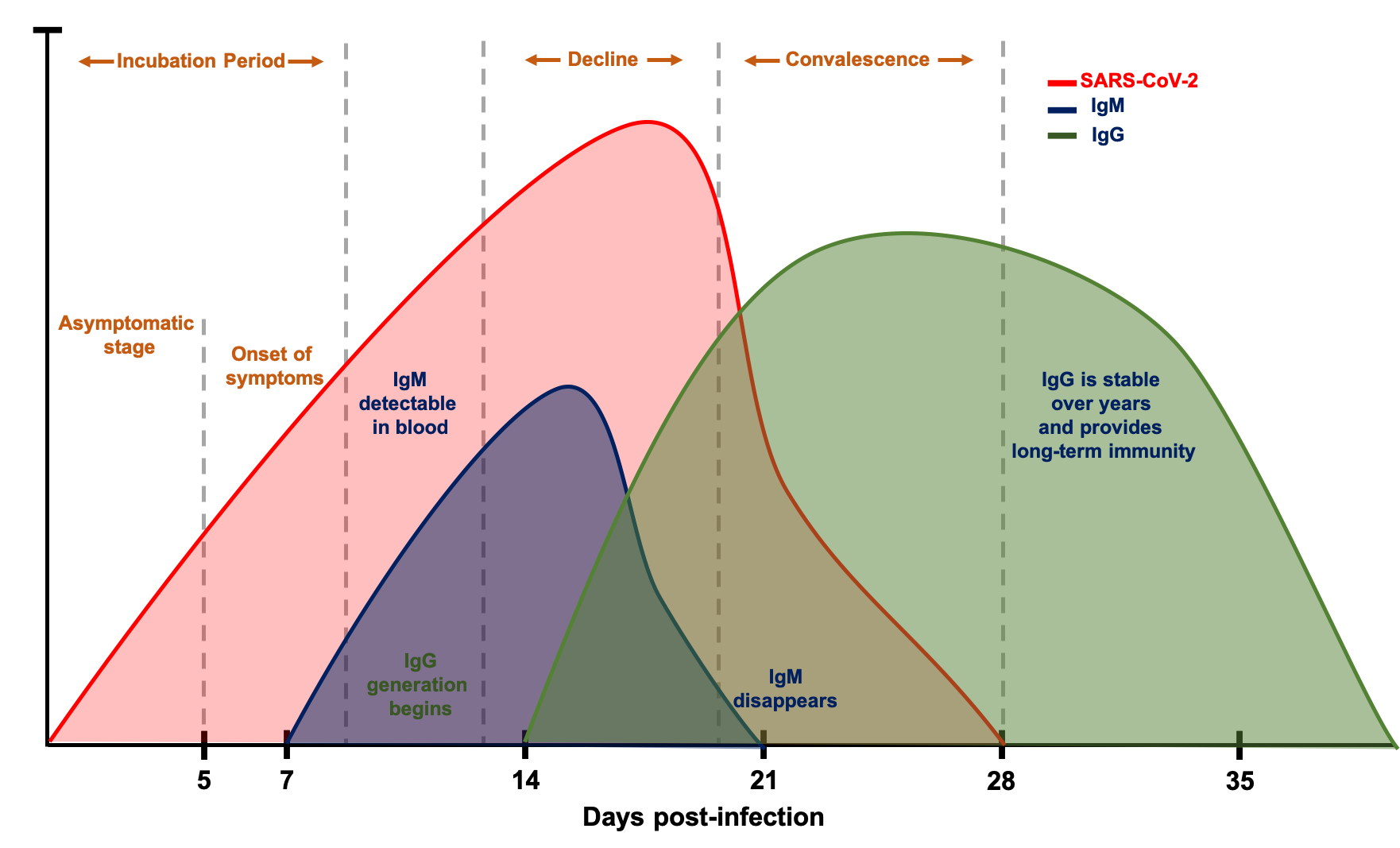
Changes in SARS-CoV-2, IgM and IgG Antibodies Following COVID-19 Infection
This chart is for illustrative purposes only
The below table summarizes the current knowledge about the course of SARS-CoV-2 genetic material, IgM and IgG antibodies, following an infection. While IgM/IgG levels offer a comprehensive view into the disease course in a COVID-19 patient, the antibodies are not detectable in the blood prior to the onset of symptoms. In other words, the IgM/IgG serological test is not ideal for early detection of COVID-19. Several scenarios for interpretation of the levels of the virus and IgM/IgG antibodies have been proposed. Individuals testing positive for all three indicates an active phase of the infection, whereas individuals testing positive for IgM only might indicate a false positive test of SARS-CoV-2. Among all the different possible scenarios, testing positive for IgG and negative for SARS-CoV-2 and IgM would indicate a convalescent individual who could safely return to work. To understand disease-stage, to stratify patients for clinical trials and to identify convalescent patients, combining RT-PCR and serological testing is crucial (16).
Treatment: Convalescent plasma therapy
On March 24, 2020, the Food and Drug Association (FDA) of the United States authorized the use of convalescent plasma as an emergency treatment for severely ill COVID-19 patients, though not as yet, for prevention (18). Convalescent plasma therapy is the transfusion of the fluid component of the blood, i.e. plasma, from individuals who recovered from the disease to patients with active infection (17). Convalescent plasma is rich in neutralizing antibodies that contribute to the clearance of the virus and therefore also to the recovery of the patient, noting that plasma from the donor should be compatible with the blood type of the recipient.
Several clinical trials are underway to assess the potential of convalescent plasma therapy in treating severely ill patients and preventing infection in high risk groups. In China, a non-controlled case series study showed that five critically ill COVID-19 patients made a full recovery following such treatment (19). In an independent feasibility pilot study, also conducted in China, 10 patients demonstrated significant improvement in clinical outcomes with minimal adverse effects a few days after receiving a single transfusion of convalescent plasma (20).
Both studies show that convalescent plasma has a potential therapeutic effect with low risk and several countries have started implementing this method to treat patients with life-threatening COVID-19 infections.
Section Contributors:
Epidemiology- Dr. Vijay Gupta and Dr. Yoshie Kobayashi, Diagnostics- Dr. Nour Majbour, Treatment- Dr. Nour Majbour, Illustrations- Dr. Yoshie Kobayashi and Dr. Nour Majbour, Edited by- Dr. Adviti Naik and Dr. Alexandra E. Butler
For references, please click here.
